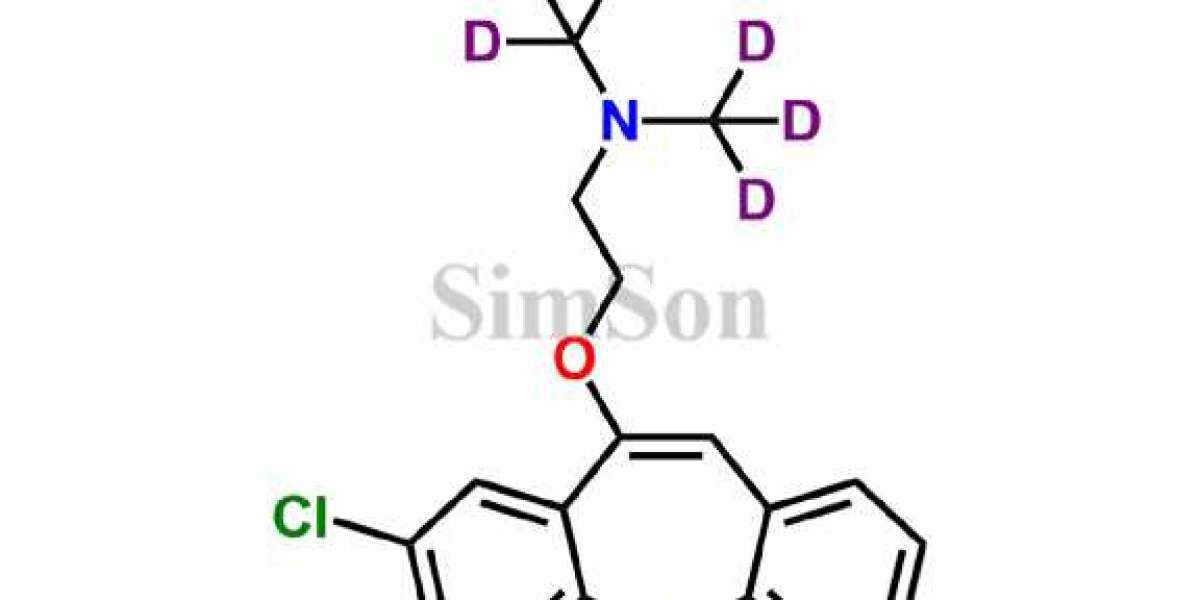
Introduction:
In the dynamic landscape of the pharmaceutical and chemical industries, the pursuit of excellence and adherence to stringent quality standards are paramount. This blog delves into the realm of impurity standard exporters, certified reference standard exporters, and API exporters, highlighting their crucial roles in ensuring the highest quality in the production of pharmaceuticals and chemicals.
Impurity Standard Exporters:
Impurity standards play a pivotal role in pharmaceutical research and manufacturing. These standards serve as benchmarks for identifying and quantifying impurities in drugs, ensuring the final product meets regulatory requirements. Impurity standard exporters specialize in providing high-quality reference materials that enable pharmaceutical companies to conduct accurate analyses, ensuring the safety and efficacy of their products.
Certified Reference Standard Exporters:
Certified reference standards are indispensable in the calibration and validation of analytical instruments, guaranteeing accurate and reliable results in laboratory testing. Certified reference standard exporters are instrumental in the supply chain, offering meticulously characterized substances that serve as reference points for various analyses. These standards are essential for maintaining consistency and reproducibility in research and manufacturing processes.
API Exporters:
Active Pharmaceutical Ingredients (APIs) form the core components of pharmaceutical formulations. API exporters play a crucial role in the global pharmaceutical supply chain by providing these essential ingredients to drug manufacturers worldwide. Adhering to stringent quality controls and regulatory standards, API exporters ensure that pharmaceutical companies receive high-quality APIs, contributing to the safety and efficacy of the final drug products.
Synergy in Quality Assurance:
Impurity standard exporters, certified reference standard exporters, and API exporters collectively contribute to the enhancement of quality assurance in the pharmaceutical and chemical industries. The collaboration between these entities ensures that pharmaceutical companies have access to the necessary tools and materials to meet regulatory requirements and deliver safe and effective products to consumers.
Global Impact:
In an interconnected global market, the impact of impurity standard exporters, certified reference standard exporters, and API exporters is felt across borders. These exporters facilitate international collaboration in research and development, ensuring that the highest standards are maintained throughout the entire pharmaceutical supply chain.
Conclusion:
As we navigate the complex landscape of pharmaceuticals and chemicals, the importance of impurity standard exporters, certified reference standard exporters, and API exporters cannot be overstated. Their commitment to quality, adherence to regulatory standards, and contribution to global collaboration make them essential players in shaping the future of the pharmaceutical and chemical industries. By elevating quality standards, these exporters pave the way for advancements that benefit both the industry and, ultimately, the well-being of consumers worldwide.