In the dynamic landscape of the pharmaceutical industry, adherence to stringent quality standards is paramount. Brazil, with its growing pharmaceutical sector, places a high premium on ensuring the safety and efficacy of drugs. Simsonpharma, a pioneering company committed to pharmaceutical excellence, is at the forefront of providing USP and EP impurity standards in Brazil. In this blog, we will delve into the significance of these standards and how Simsonpharma is contributing to the pharmaceutical ecosystem in the country.
Understanding USP and EP Impurity Standards:
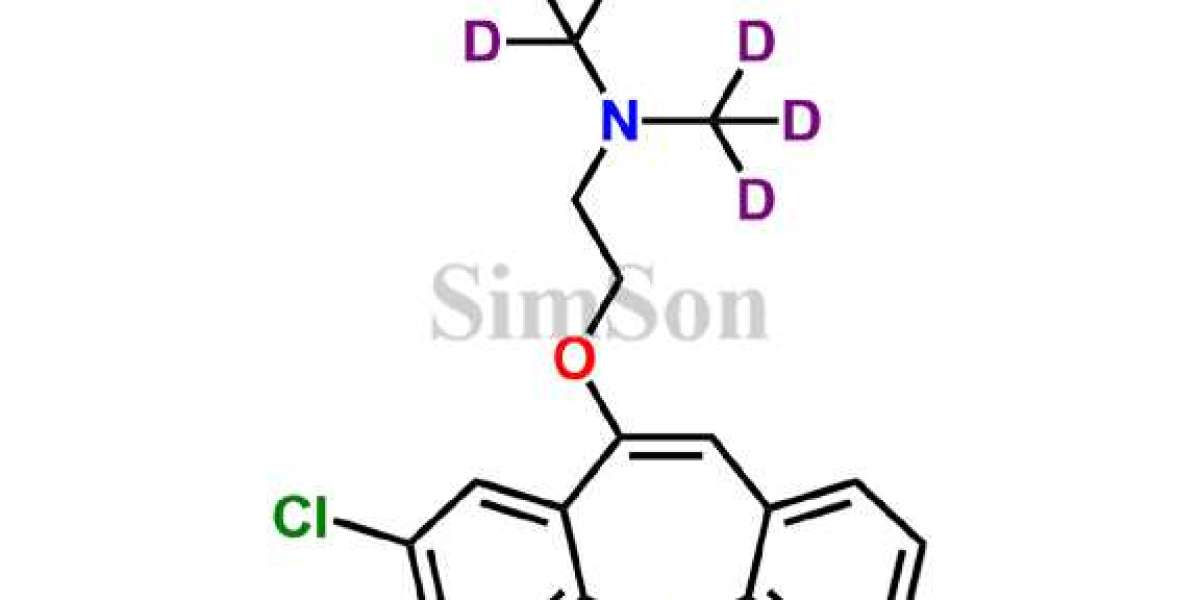
The United States Pharmacopeia USP standards in Brazil and European Pharmacopoeia (EP) are globally recognized compendia that establish quality standards for pharmaceutical ingredients, dosage forms, and related products. These standards ensure consistency, quality, and safety in the production and distribution of medicines.
Simsonpharma's Commitment to Quality:
Simsonpharma stands out as a reliable source for USP and EP impurity standards in Brazil. The company's unwavering commitment to quality is reflected in its comprehensive range of standards that comply with international regulations. Whether it's meeting the USP standards for purity or the EP standards for impurities, Simsonpharma provides pharmaceutical companies in Brazil with the assurance they need for their products.
Drug Impurity Standards in Brazil:
In addition to USP and EP standards, Simsonpharma is a trusted supplier of drug impurity standards in Brazil. Drug impurities can significantly impact the safety and efficacy of pharmaceutical products. Simsonpharma's portfolio includes a diverse range of meticulously characterized impurity standards, enabling pharmaceutical companies to conduct thorough quality control and ensure regulatory compliance.
Why Choose Simsonpharma:
Unparalleled Quality Assurance: Simsonpharma's USP and EP impurity standards in Brazil meet the highest quality benchmarks, ensuring the reliability and accuracy of test results.
Regulatory Compliance: Staying ahead of regulatory requirements is crucial in the pharmaceutical industry. Simsonpharma's standards help companies in Brazil adhere to local and international regulations.
Custom Solutions: Recognizing the diverse needs of pharmaceutical manufacturers, Simsonpharma offers customized solutions, tailoring impurity standards to specific product requirements.
Expert Support: Simsonpharma's team of experts provides guidance and support throughout the entire process, from selecting the right standards to addressing any technical queries.
Conclusion:
Simsonpharma emerges as a beacon of excellence in the realm of pharmaceutical standards in Brazil. By offering a comprehensive suite of USP, EP, and drug impurity standards, the company plays a pivotal role in safeguarding the integrity of pharmaceutical products. As Brazil's pharmaceutical industry continues to evolve, Simsonpharma stands as a steadfast partner, empowering companies to uphold the highest standards of quality and compliance. Elevate your pharmaceutical endeavors with Simsonpharma – where quality meets excellence.