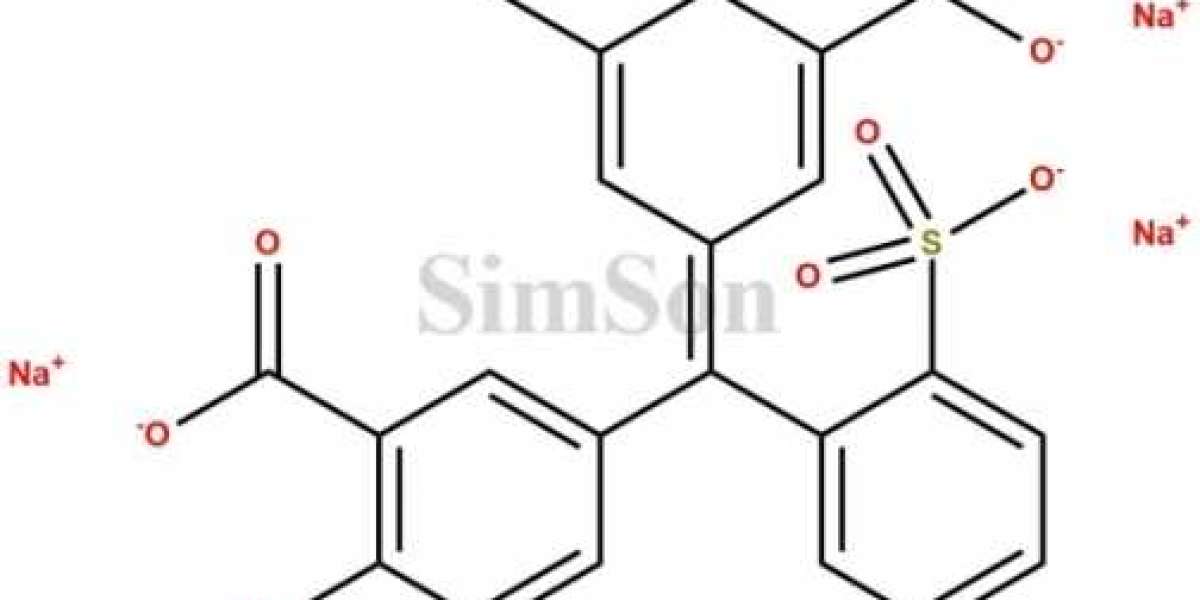
In the pharmaceutical industry, maintaining rigorous standards of quality and purity is paramount to ensuring the safety and efficacy of medications. As a leading player in the global pharmaceutical landscape, Brazil is home to renowned manufacturers and suppliers of impurity standards and certified reference standards. Among these trusted names stands Simsonpharma, a distinguished company dedicated to upholding the highest standards of excellence. In this blog, we'll explore the significance of impurity standards, certified reference standards, and how Simsonpharma plays a vital role in elevating pharmaceutical standards in Brazil.
Impurity Standard Manufacturers Brazil: Setting the Benchmark
Impurity standards are essential tools used in pharmaceutical analysis to assess the purity and quality of drug substances and products. In Brazil, a select group of manufacturers specializes in producing high-quality Impurity Standard Manufacturers Brazil that meet stringent regulatory requirements. Simsonpharma is at the forefront of this industry, renowned for its precision and reliability in manufacturing impurity standards that adhere to international pharmacopeial standards. Pharmaceutical companies across Brazil trust Simsonpharma's impurity standards for their accuracy and consistency, ensuring the integrity of their products.
Certified Reference Standard Suppliers Brazil: Ensuring Accuracy and Consistency
Certified reference standards are indispensable for the accurate quantification and identification of drug substances in pharmaceutical analysis. In Brazil, certified reference standard suppliers Brazil play a vital role in supplying these critical materials to pharmaceutical laboratories and research institutions. Simsonpharma stands out as a trusted supplier of certified reference standards in Brazil, offering a comprehensive range of meticulously characterized materials that meet the highest quality standards. With Simsonpharma's certified reference standards, pharmaceutical professionals can confidently perform precise and reliable analyses, ensuring the safety and efficacy of pharmaceutical products.
Impurity Standards Suppliers Brazil: Meeting Regulatory Requirements with Confidence
As pharmaceutical regulations continue to evolve, compliance with stringent quality standards remains a top priority for companies operating in Brazil. Impurity Standards Suppliers Brazil are indispensable tools for ensuring compliance with regulatory requirements and demonstrating the quality and safety of pharmaceutical products. Simsonpharma serves as a reliable partner for pharmaceutical companies in Brazil, offering a wide range of high-quality impurity standards that meet regulatory specifications. With Simsonpharma's impurity standards, pharmaceutical manufacturers can navigate regulatory challenges with confidence, ensuring the integrity of their products and the safety of consumers.
Conclusion:
In Brazil's dynamic pharmaceutical landscape, maintaining high standards of quality and purity is essential for ensuring the safety and efficacy of medications. Simsonpharma stands as a beacon of excellence, providing pharmaceutical companies across Brazil with reliable impurity standards and certified reference standards. With Simsonpharma's unwavering commitment to quality and precision, pharmaceutical professionals can trust that their products meet the highest standards of excellence, safeguarding public health and advancing the pharmaceutical industry in Brazil.